We live in the era of portability; cords and wires are used less and less for powering up devices and consumer electronics (laptops, mobile phones, toothbrushes, torches, etc.). We also live in the era of ‘e-mobility’ or electrification. Electric vehicles (EVs) are the highly anticipated mobility alternative for a zero-emission, greener future.
Current and future battery applications
Battery innovation is significantly driven by the development of batteries for EVs, which must be long-lasting, safe, fast charging and, of course, provide sufficient distance between charges. EVs include not only cars, but also bikes, scooters, buses, trucks (light & medium duty) and boats. Projects to make planes electric are also in progress.
Besides mobility, batteries are key to energy storage. They open the way to harvest renewable energy sources (solar, wind, hydro, biomass, tidal, and geothermal energy). The cost for utility-scale battery storage in the US dropped 70% (from $2,152/kWh to $625/kWh) between 2015 and 2018 (US Energy Information Administration). It is expected to drop even lower (another 45%) by 2030 (US National Renewable Energy Laboratory).
However, there are challenges in battery design which must be overcome in order for this zero-emission future to materialise. The challenges to develop suitable batteries are different at environmental temperature extremes. Ongoing experimentation with new materials, intending to improve efficiency and tackle known issues, adds new complexity to the mix as battery materials are required to have predictable behaviour for maximum safety.
Spent EV batteries can be used in second life, less demanding applications compared to EVs (grid energy storage) and, eventually, can be recycled; contributing, thus, to competitive sustainability and circular economy as well as ensuring that materials stay in circulation rather than being constantly mined and only to be discarded after a single use.
So, how can batteries become more powerful and reliable?
Everything starts with effective material characterisation, from raw materials mining to the final product. The materials destined to become battery components are required to be free of impurities or any other accidentally introduced contamination which could undermine materials performance or compromise safety.
Energy dispersive spectroscopy (EDS) is a quick, non-destructive, and non-invasive technique, with high sample throughput – ideal for performing efficient material screening. The electrode precursor material is in powder form. For cathodes, this powder is commonly a mix of nickel, cobalt and manganese (NCM); aluminium is also often included.
There is a tendency to replace cobalt due to cost fluctuations and controversial mining practices. New compositions are constantly being tested to find the optimal recipe. The quality assurance and control of these powders is essential to ensuring material performance and lifetime. AZtecBattery™ offers an automated SEM-EDS system for controlling powder composition and detecting contamination. There are many benefits to using AZtecBattery:
1. automation - no instrument downtime
2. reliable characterisation (particle morphology, size, composition, count)
3. morphological and compositional characterisation of >120,000 particles/hour with Ultim[SA2] Max
4. particle reconstruction for large particles extending to multiple fields of view
5. high sample throughput
6. customisable preset classification schemes
7. user profiles: sharing analytical profiles across sites for enhanced consistency and better SOPs
8. user-friendly interface with navigators and step notes to help new users
9. custom results reporting
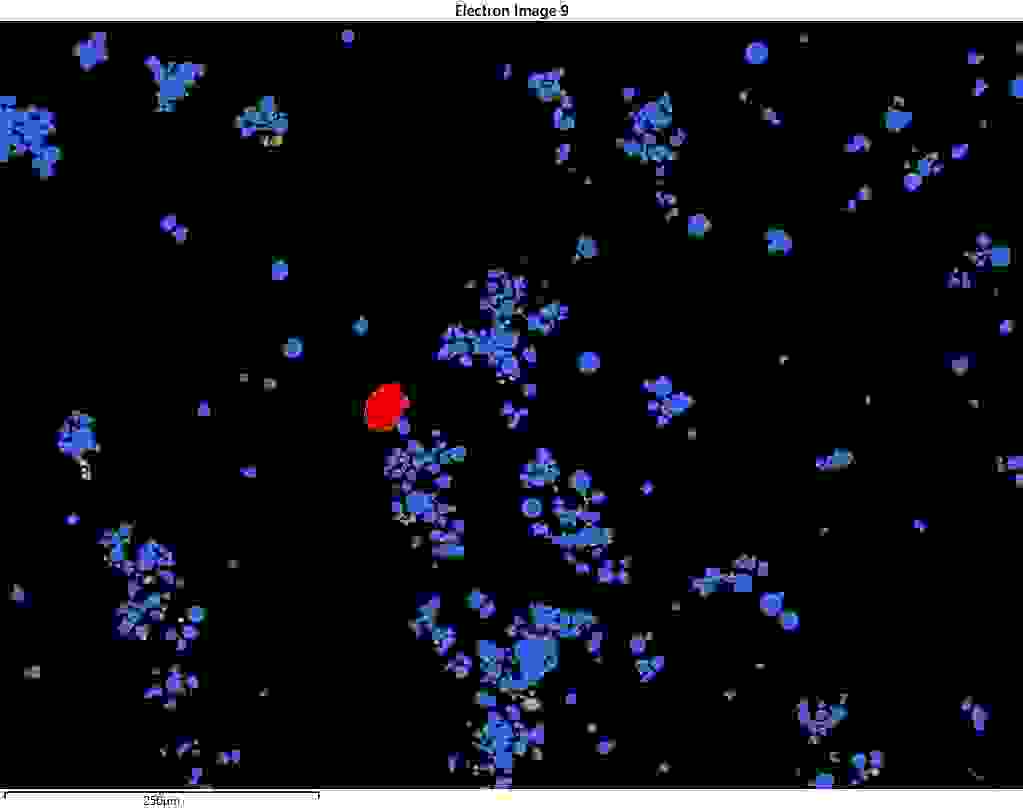
Figure 1: Contamination particle (in red) in NCM811 battery particles (blue).
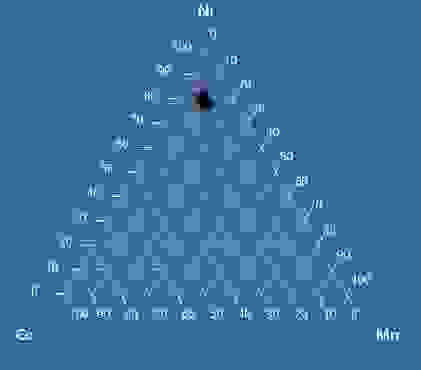
Figure 2: Ternary diagram of NCM811 (precursor for cathodes) battery powder composition.
Analysis of beam sensitive materials in the SEM
Battery materials are often beam sensitive. In that case, a low beam accelerating voltage is recommended to avoid damaging the sample. If the sample suffers beam damage, then while the analysis is accurate, it will reflect the beam damage on the sample (secondary) and not the primary map composition. Such an example is presented in the images below. Beam damage is obvious with secondary phases forming on the sample surface in an attempt to analyse lithium.
Figure 3 Left: Solid state garnet electrolyte before analysis. Right: Same material analysed at 5 kV 500 pA for 10 minutes. The sample suffered significant beam damage resulting in Li exfoliating from the bulk, forming dendrites on the surface.
Ultim Extreme is a windowless EDS detector designed to operate at low accelerating voltage and produce high resolution images. It is a sensitive and powerful detector and is the first EDS detector that is capable of consistent detection of elements as light as lithium. As such, its application range extends from life sciences to materials science and includes battery research and development.
Oxford Instruments can help with applications ranging from manufacturing to advanced R&D.
References
Burgess, Simon, Xiaobing Li, and James Holland. "High spatial resolution energy dispersive X-ray spectrometry in the SEM and the detection of light elements including lithium." Microscopy and Analysis 6 (2013): S8-S13.
Hovington, P., Timoshevskii, V., Burgess, S., Demers, H., Statham, P., Gauvin, R., & Zaghib, K. (2016). Can we detect Li KX‐ray in lithium compounds using energy dispersive spectroscopy?. Scanning, 38(6), 571-578.
Reed, S. (2005). Electron Microprobe Analysis and Scanning Electron Microscopy in Geology (2nd ed.). Cambridge: Cambridge University Press. doi:10.1017/CBO9780511610561Links
https://www.bbc.com/future/article/20201217-renewable-power-the-worlds-largest-battery
https://www.eia.gov/todayinenergy/detail.php?id=45596&src=email






