Electrolyte and Separator Research
In lithium-ion batteries the cathode and anode are separated by a separator membrane which contains the usually liquid electrolyte. Alternatively, solid electrolytes are being researched which could offer meaningful improvements in safety and energy density as well as offer cost savings.

Liquid electrolytes
The most commonly used liquid electrolyte is lithium-salt solution (LiPF6 or LiBF4) with binders and additives added to reduce gassing and increase the viscosity. Liquid electrolytes can degraded and outgas during the charge and discharge process which can lead to swelling of the battery and for some reaction products can also lead to corrosion of the battery. For example, hydrofluoric acid can be formed as a breakdown product.
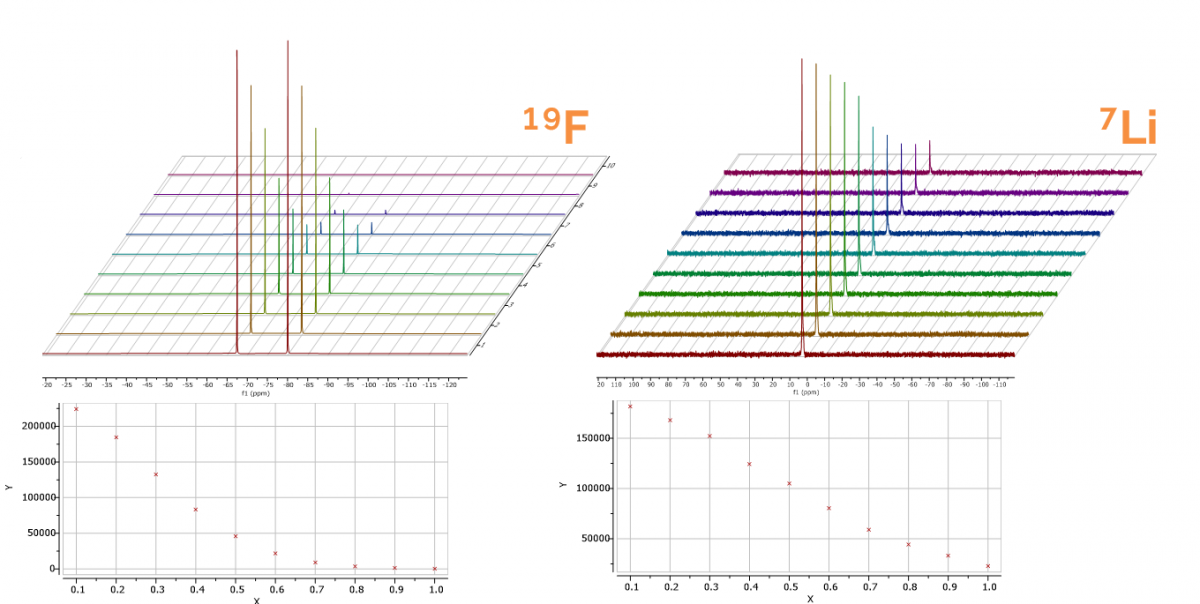
Oxford Instruments cryogen-free X-Pulse NMR system is the only benchtop NMR that can measure all components in a single system (1H, 13C, 19F, 7Li, 31P, 11B). It also reliably identifies and quantifies breakdown products such as HF and LiF and measure diffusion coefficients.
Figure 1 (right): The X-pulse benchtop NMR system comes with pulsed field gradients as standard. This allows measurement of self diffusion, like those shown, to understand key physical properties of the electrolyte, including the conductivity and the ion transference.

Solid electrolytes
A number of different materials are being researched as candidates for solid electrolytes in Li-ion batteries which are safer but also potentially higher energy density than existing batteries by enabling the use of metallic lithium anodes. However, the conductivity of Li ions is usually lower in solid state electrolytes than in their liquid counterparts. Imaging the Li distribution in the SEM can provide vital information about the materials structure as well as any detrimental dendrite formation. In combination with conductivity measurements it enables new research aiming to improve the performance of solid-state electrolytes and eventually enable an all solid-state battery to be built. Ultim Extreme is the only EDS detector that enables the detection of lithium X-rays in an SEM at low enough beam currents to not significantly alter the sample.
Figure 2: (Left) Solid State Garnet Electrolyte before acquisition; (middle) X-ray map
acquired at 2.5kV with Ultim Extreme at 100pA beam current – three different Li containing
phases identified; (right) Same area after acquisition with no observable damage
Related Products